- Welcome to Shanghai Yuzhi Technology Co., Ltd. official website!
- CN
- Hotline:0571-63550890
The adiabatic method is based on the Thermodynamic state of a substance whose energy is recovered to the same. Take a sample of a certain mass, heat it to a constant temperature under a certain pressure, and then put it into a calorimeter with a constant temperature. The rise of the calorimeter temperature is positively related to the energy, thus indirectly calculating the specific heat/enthalpy of the substance.
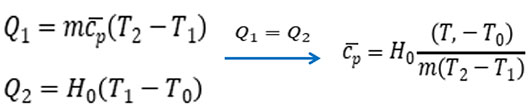
1. The substance is sealed in the crucible, which can effectively prevent sample corrosion, overflow, volatilization, and other issues;
2. Steady state measurement with high measurement accuracy.